On July 3, 2020, after a second review on the draft amendments to the Patent Law, the Standing Committee of the National People’s Congress (SCNPC), published the draft amendments and invited public opinion on the draft amendments until August 16, 2020. This is an important legislative development in the implementation of the Phase One Trade Agreement between the United States and China.
Most notably, in Article 75 of the current draft amendments, following the Bolar Exception clauses, several new paragraphs were added, which are believed to form the framework for the upcoming drug-related artificial patent infringement in China.
Proposed artificial patent infringement and declaratory judgment
Proposed Paragraph 2 of Article 75 provides that if the patentee or interested party believes that a drug in a market approval application falls within the scope of patents listed on the China Patent Information Registration Platform for Listed Drugs, the patentee or interested party may file a lawsuit with the court or apply for an administrative ruling with China National Intellectual Property Administration (CNIPA) within 30 days from the date the National Medical Products Administration (NMPA) announces the application for market approval. If the patentee or interested party fails to file such a lawsuit or request, the applicant for market approval of the drug may request the court or CNIPA to confirm that the relevant drug does not fall within the scope of patents listed on the China Patent Information Registration Platform for Listed Drugs.
This provision provides a basis for early resolution of potential patent disputes due to the filing of a market approval application with NMPA. Item (5) of Paragraph 1 of Article 69 of the current Chinese Patent Law prescribes the Chinese Bolar exception, which is similar to the US Bolar exemption, and was used as a basis for market approval of infringing products in China. Due to the lack of an artificial patent infringement regime, this Bolar exception has long been criticized as the “Naked Bolar exception,” which makes it difficult for drug innovators to safeguard their rights. Take the year 2019 for example: more than 10 generic drugs obtained drug approvals (nearly one approval per month), while the related patents of brand name drugs were still valid.
Now with the proposed artificial patent infringement regime, the current Bolar exception under Chinese Patent Law will be restricted to preparation work before making market approval application with NMPA, i.e., making, using or importing patented drugs, whereas submitting approval applications with NMPA will become a cause of action for patent infringement.
Compared with artificial patent infringement in the US, there are two significant differences in this proposed artificial patent infringement regime. One is the administrative approach to the artificial patent infringement disputes, which is a unique Chinese character, and an efficient way for early resolution of potential patent disputes, especially considering the heavy workload faced by IP courts and IP tribunals in China.
The second significant difference is the option for the applicant for market approval to seek a declaratory judgment, in case that the patentee or interested party does not want to initiate the artificial patent infringement action and have an early resolution to the potential patent disputes. There might be cases where the patentee or interested party wants to obtain monetary compensation from patent infringement and therefore intentionally chooses to keep silent before the regulatory approval of generic drugs and wait for a later “harvest” after launch of the generic drugs. This is apparently against the original purpose of the patent linkage system and may severely influence the drug accessibility for which NMPA and the China central government care most.
Nine-month limit for concluding an artificial patent infringement dispute
Proposed Paragraph 3 of Article 75 provides that the court or CNIPA should make an effective decision or administrative ruling within nine months from the date on which the request of the patentee or interested party is accepted. For market approval applications that have passed the NMPA technical review, NMPA may make a decision on market approval based on the decision of the court or the administrative ruling of CNIPA. If the party concerned is not satisfied with the administrative ruling of CNIPA, it may file a lawsuit with the court within 15 days from the date of receiving the administrative ruling.
According to the procedural laws, nine months is exactly the time limit for concluding a lawsuit through the Chinese two-tier trial system. That is, six months for the first instance (trial stage), and an additional three months for the second instance (appellate stage). But due to the heavy workload in front of the IP courts and IP tribunals around the country, only a few cases can actually be concluded within nine months. The first instance per se may take more than one year. As to administrative rulings, although it is possible for CNIPA to work out a ruling within four months or even less time, if one party is unsatisfied with the ruling and appeals the ruling to the court, it would be very difficult to conclude all three procedures (CNIPA procedure, trial stage and appellate stage) within nine months. Therefore, this nine-month limit is very ambitious, it shows the determination of decision makers and requires priority processing of drug approval related cases in the judicial system.
Reassuringly, the second sentence in this paragraph indicates that it is not necessary for NMPA to wait for an effective decision of the court, which is generally an outcome of the second instance. Considering the not-so-complex nature of drug-related patent infringement assessment and the lengthy time needed for concluding a lawsuit through judicial system, it makes sense to allow NMPA to give green light based on a first instance decision or an administrative ruling, because the original purpose of artificial patent infringement regime is early resolution of disputes.
That is to say, the nine-month limit here is not a time period for NMPA to suspend the market approval application and therefore does not equal to the 30-month stay period in the US patent linkage system. Also, in the proposed provisions, there is no mention about issuance of a provisional approval by NMPA if an effective infringement decision is not obtained within nine months. And a reasonable estimate is there won’t be such a provisional approval. NMPA may grant market approval for generic drugs before receiving a final (effective) decision from the court, so long as there is a court decision or an administrative ruling. In this way, we can expect a more efficient and faster dispute resolution under the proposed China patent linkage system.
Considering the time efficiency offered by this artificial patent infringement regime, we cannot expect 100 percent certainty of its outcome at the same time. For those generic manufacturers who are eager to launch their drugs before seeing a final decision, it is fair for them to also take the risk of a later infringement judgement.
NMPA is working with CNIPA on more details of the Chinese patent linkage system
Proposed Paragraph 4 of Article 75 mentions that NMPA shall work with CNIPA jointly to formulate specific linkage measures for drug market approval and resolution of patent disputes at the stage of market approval applications, and shall implement such measures after being approved by the State Council.
The above newly-added provisions merely set forth the framework of China’s artificial patent infringement regime, and more details remain to be addressed in implementing regulations of the Patent Law, judicial interpretations and administrative measures. Among other details, the establishment of the “China Patent Information Registration Platform for Listed Drugs” and clarification on the declaration obligation of market approval applicants are the ground works and most urgent tasks in front of NMPA and CNIPA.
According to the latest estimation, NMPA and CNIPA may be able to work out these implementing regulations and administrative measures in October 2020. In the meanwhile, the Supreme Court is now busy working on the relevant judicial interpretation, i.e., Provisions on Issues Concerning the Application of Law in the Trial of Cases of Drug Patent Link Disputes, which is to be completed by the end of 2020 according to the legislative plan of the Supreme Court.
With the finalization of the draft amendments to the Patent Law after a third review by the Standing Committee of the National People’s Congress and the establishment of detailed implementing regulations, judicial interpretations and administrative measures, we are going to witness the birth of a patent linkage system of Chinese character and its significant impacts on pharmaceutical industry in China.
Suggestions for branded companies and drug innovators
In view of the emerging patent linkage system and rapid legislative progress in China, we suggest branded companies and drug innovators to closely monitor the development of legislative process, and get the necessary preparation work done in advance.
For branded companies and drug innovators, necessary preparation works includes:
Get familiar with NMPA’s Catalogue of Approved Drug Products and be prepared for the China Patent Information Registration Platform for Listed Drugs. On December 28, 2017, NMPA issued the first edition of Catalogue of Approved Drug Products, which covers both innovative and generic drugs and was called the Chinese Orange Book. Patents on compounds, salts of compounds, formulations, dosage forms and new indications are allowed to be registered in this catalogue. It is estimated that relevant provisions on the Catalogue of Approved Drug Products will also apply to the proposed China Patent Information Registration Platform for Listed Drugs.
Branded companies and drug innovators should carefully review their own patent portfolio and elect the suitable patents for the coming patent registry with NMPA.
Closely monitor the R&D activities and market approval applications of generic companies and prepare necessary documents in advance. According to the current draft, the patentee or interested party only has a 30-day response period from the date NMPA announces the application for market approval, which is very short for sufficient preparation.
Branded companies and drug innovators should select qualified lawyers in advance and get necessary empowering documents executed before they are actually needed. For foreign right holders, these documents may need to be notarized and legalized, which may take more than 30 days.
When initiating the artificial patent infringement action, a patent infringement analysis would be a must requirement. In this connection, a patent infringement assessment opinion made by a Chinese law firm or a judicial appraisal centre would be helpful for the court or CNIPA to make a quick decision. As such, it is recommended to have such a patent infringement analysis at hand, no matter who is the potential infringer.
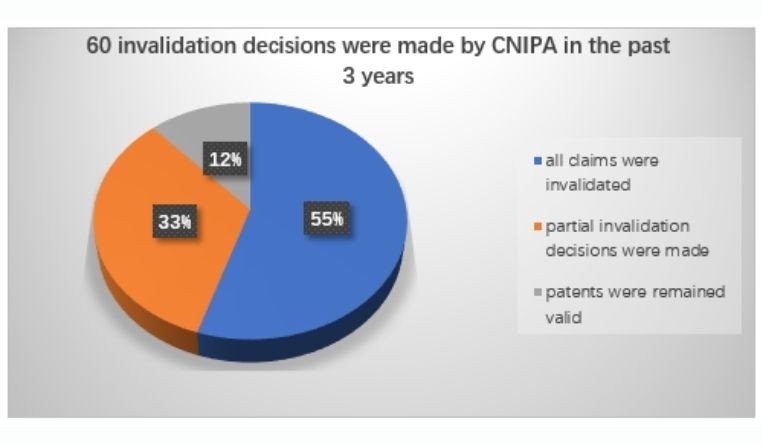
Be prepared for a surge of patent invalidation actions. According to the latest statistics, in the past three years (2017-2019), patents on 38 innovative drugs were challenged in China by generic companies. Sixty invalidation decisions were made by CNIPA, wherein in 33 cases (55%) all claims were invalidated, in 20 cases (33%) partial invalidation decisions were made, and only in seven cases (12%) the patents remained valid. In these invalidation cases, lack of inventiveness (Article 22.3) is the most commonly used invalidation ground.